|
Forums36
Topics40,947
Posts557,814
Members18,485
| |
Most Online3,612
Jan 10th, 2023
|
|
|
|
Joined: Jun 2008
Posts: 9
Fingerling
|
OP

Fingerling
Joined: Jun 2008
Posts: 9 |
This is the first time I have tested the ph and its way up at 9.2 I am getting ready to put in a bunch of HSB and don't want sick or dead fish.
When the pond was built we put 3 ten wheeler loads of concrete pieces in 3 piles for cover. they are big pieces but concrete all the same. Could the concrete be leaching too much lime? How long will the concrete leach lime?
Any recomendations appreciated.
The pond is about 1 acre and holds about 1.4 mil. Gal.
|
|
|
|
|
Joined: Mar 2005
Posts: 21,490 Likes: 265
Moderator Hall of Fame 2014  Lunker
|

Moderator Hall of Fame 2014  Lunker
Joined: Mar 2005
Posts: 21,490 Likes: 265 |
ph varies a lot over a 24 hour period. You need to check the alkalinity. It would take some very acid water (in fish terms) to dissolve much concrete. As ground- or rainwaters flow over and percolate through soil and underground rock formations containing calcitic limestone (CaCO3) or dolomitic limestone [CaMg(CO3)2], the acidity produced by CO2 will dissolve limestone and form calcium and magnesium bicarbonate salts: http://srac.tamu.edu/index.cfm?catid=25http://srac.tamu.edu/getfile.cfm?pubid=112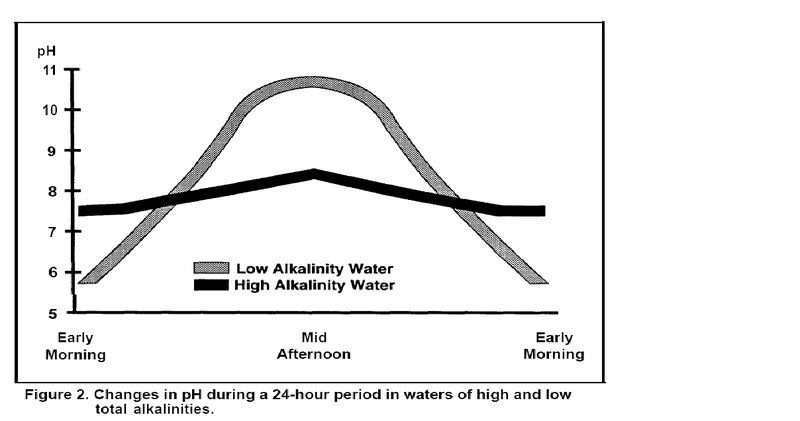 
Last edited by ewest; 03/30/09 08:55 AM.
|
|
|
|
|
Joined: Aug 2002
Posts: 20,043 Likes: 1
Hall of Fame  Lunker
|

Hall of Fame  Lunker
Joined: Aug 2002
Posts: 20,043 Likes: 1 |
Not all waters have that diurnal PH shift though Eric. My ponds don't change at all due to the alkalinity that is almost off the scale that of course acts as a buffer.
If pigs could fly bacon would be harder to come by and there would be a lot of damaged trees.
|
|
|
|
|
Joined: Mar 2005
Posts: 21,490 Likes: 265
Moderator Hall of Fame 2014  Lunker
|

Moderator Hall of Fame 2014  Lunker
Joined: Mar 2005
Posts: 21,490 Likes: 265 |
Cecil that is noted in the chart (dark line - high alkalinity - little change). No way to know that without repeated ph tests over an extended time or an alkalinity test. IMO if he has super high alkalinity it is from a source other than the concrete as it takes high acid water (in fish terms) to break down the concrete. My guess is low alkalinity water with high ph swings due to low buffering is the cause of the 9.2 reading and it is not caused by the concrete.
sdstohler what time of day was the ph test done and was it a clear sunny day ?
|
|
|
|
|
Joined: Jun 2008
Posts: 9
Fingerling
|
OP

Fingerling
Joined: Jun 2008
Posts: 9 |
It was an overcast day with about a 5 mph wind. its the only ph test done. I have to get a tester of my own so I can do tests often. The desolved oxygon was 110 also. I haven't yet put in an airator. also trout I put in are light in color . Pond guy tells me thats from photo cells in there skin and that as I develop more dark vegitation in the pond that will change. the pond is only 8 months old and only full to the banks for6 months.
|
|
|
|
|
Joined: Jun 2008
Posts: 9
Fingerling
|
OP

Fingerling
Joined: Jun 2008
Posts: 9 |
time was about 5 pm est and i'm in Pa.
|
|
|
|
|
Joined: Mar 2005
Posts: 21,490 Likes: 265
Moderator Hall of Fame 2014  Lunker
|

Moderator Hall of Fame 2014  Lunker
Joined: Mar 2005
Posts: 21,490 Likes: 265 |
Can you tell us about the land in the area and the drainage ?
|
|
|
|
|
Joined: Aug 2002
Posts: 20,043 Likes: 1
Hall of Fame  Lunker
|

Hall of Fame  Lunker
Joined: Aug 2002
Posts: 20,043 Likes: 1 |
Not all waters have that diurnal PH shift though Eric. My ponds don't change at all due to the alkalinity that is almost off the scale that of course acts as a buffer. My bad I scanned the chart too quickly! 
Last edited by Cecil Baird1; 03/31/09 08:30 PM.
If pigs could fly bacon would be harder to come by and there would be a lot of damaged trees.
|
|
|
|
|
Joined: Jun 2008
Posts: 9
Fingerling
|
OP

Fingerling
Joined: Jun 2008
Posts: 9 |
Lets see, We are at the beginning of the delaware watershed. in fact runoff from 1 side of rt 897 goes to the susquhanna and on my side of the street runoff goes to the delaware. The collection area that feeds the pond is relatively small but there is some street runorff in it. maybe over 1/2 a square mile. not many homes in the mix. maybe 4 or 5.
Its heavy clay soil but the clay is under 18 inches of topsoil. topsoil that stays wet and pretty sticky for days after a rain. it drys rock hard when it dries. The bedrock as far as i know is blue shale and with a mix of limestone. they tell me that drilling wells here is difficult because of voids in the shale , like honeycome.
when we dug the pond it was just about 12 ft before we hit a rock and all clay. 1 spring in the bottom very slow feed from that . the water from that spring was clear. the clay and rock blue gray. my water had a green tint for a long time but is very clear now. have a nice alge bloom going but there is not much vegetation in the pond its self. What is there looks healthy and is growing. Thanks again
|
|
|
|
|
Joined: Mar 2005
Posts: 21,490 Likes: 265
Moderator Hall of Fame 2014  Lunker
|

Moderator Hall of Fame 2014  Lunker
Joined: Mar 2005
Posts: 21,490 Likes: 265 |
I suggest you contact the people below and have the soil and water tested. My guess is you have high alkalinity due to water/spring going through limestone. Penn. ---- Agricultural Analytical Services Penn State University Tower Rd. University Park, PA 16802 (814) 863-0841 http://www.aasl.psu.edu You can purchase a soil sample collection kit at your local Penn State Cooperative Extension County Office—you'll find the list of county offices on the lab's Website or you can call the lab for your county's office. You then mail your sample directly to the lab. The results will be sent to you with a copy to your local extension agent.
|
|
|
|
|
Joined: Jun 2008
Posts: 9
Fingerling
|
OP

Fingerling
Joined: Jun 2008
Posts: 9 |
|
|
|
|
|
Joined: Mar 2005
Posts: 21,490 Likes: 265
Moderator Hall of Fame 2014  Lunker
|

Moderator Hall of Fame 2014  Lunker
Joined: Mar 2005
Posts: 21,490 Likes: 265 |
|
|
|
|
|
Joined: Jan 2009
Posts: 10,458 Likes: 2
Ambassador
Field Correspondent
Hall of Fame Lunker
|

Ambassador
Field Correspondent
Hall of Fame Lunker
Joined: Jan 2009
Posts: 10,458 Likes: 2 |
I graduated from Penn State. Great group of guys there. The analysis is great. I have used it many times for our foot plots on our hunting property in PA.
|
|
|
|
|
Joined: Jan 2009
Posts: 17
Lunker
|

Lunker
Joined: Jan 2009
Posts: 17 |
Unless the concrete you added to your pond was allowed to soak in water for an extended amount of time (up to 4 weeks), then it will raise the pH of your pond. I used to build concrete coral substrate, and the standard practice after creating a sizeable piece of concrete was to put it in a stream or lake for a month to let the pH neutralize.
|
|
|
|
|
Joined: Feb 2006
Posts: 210
Lunker
|

Lunker
Joined: Feb 2006
Posts: 210 |
I agree with Erik. The lime stone is the cause of the high pH. It will have low buffer capacity due to "lime softening" effect (calcium bicarbonate to calcium carbonate precipitation). Lowering pH will require adding acid. Suggest addiing sulfur to the water to lower pH and alkainity.
Mike
|
|
|
Moderated by Bill Cody, Bruce Condello, catmandoo, Chris Steelman, Dave Davidson1, esshup, ewest, FireIsHot, Omaha, Sunil, teehjaeh57 |
|