I recently acquired all of the testing supplies I thought I may need to monitor changing conditions.
Yesterday I tested for Alkalinity and here's what I have..
WT: 47F
pH: 7.4
KH: 7.5= meq/L 2.678= dKH 8.008= ppm: 143
I understand pH, and 143ppm but not sure I understand what the 143ppm Alkalinity truly "means"..
I read 50-200ppm is best range, yet 20 is sometimes found as acceptable (I read).
I know this probably won't stay up long on recent posts but if anyone can tell me if these numbers "indicate" anything in particular, I'd appreciate understanding more about this.
Thanks
Google around for the science....its easy to find....but your pH reading is great as is your alkalinity.
I check both 2 or 3 times each year. The Alkalinity in our 3 BOWs is always anywhere from 70 to 145....that range is perfect. All three ponds measure pretty close to the same alkalinity levels every time, year in and year out...within 10-15 "points".
We always think of those 2 as the most important water analysis tests you can do...and they are easy to do with reasonably priced equipment (get something better than color test strips). BM61.
See this --
https://srac.tamu.edu/serveFactSheet/11220 Alkalinity is the low end of what will support a fertilizer program. It is still low. 200 is optimum.
143 is very good. 7.4 is optimum pH so 7.4 is very good.
Snipe, I envy you! In east Texas we have naturally acidic, low alkalinity conditions & must spread aglime to amend. I've added a total of about 50 tons to my pond.
I've always wondered about the correlation between Alk and PH. I added 500 pounds of aglime last spring and that only brought my Alk to 40. My hardness is still zero, but my PH stays around 7.4. I use a litmus stick to check Alk (which also checks PH and Hardness), but a 5 drop tester to check PH as well and the two are always at least close on the PH test.
I wish I could get my hardness up a little. Planning to throw more aglime in the near future.
Mike I think if you keep adding ag lime you will get Alk and Ph up. Can't add too much really, so add 500-1000 and see what happens. I added 6.6 tons my 1.8 acres and it increased Ph from 7.2 to 8.5 and Alk went up a good bit too but can't remember how much.
Keep in mind that pH swings during the day/night period. Alkalinity changes slowly and is the buffer for pH swings.
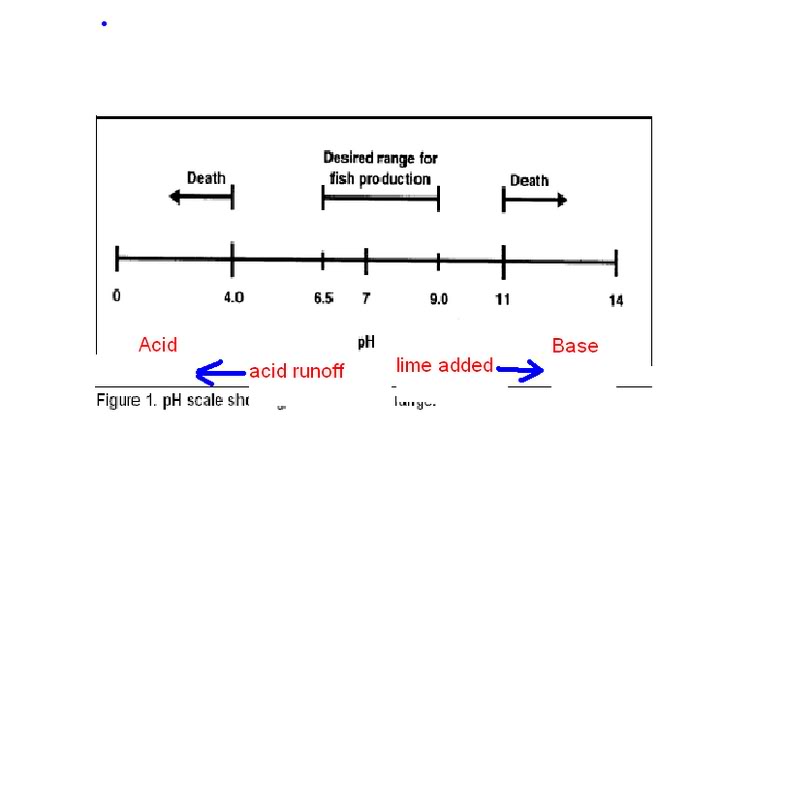

Those 2 charts pretty much tell you everything you need to know about pH and alkalinity. Thanks for giving us the visual Eric. BM61.
I've checked my PH at all hours of the day and it seldom ever gets above 8. I take samples from the same location from elbow deep water so my samples are consistent. Its stays pretty neutral, even on very bright days. I know I need more aglime, but I'll only do about 500 pounds at a time. My main quandary is why my hardness is so low.
Not much naturally occurring calcium carbonate in your watershed????? Maybe... You should be able help fix with more ag lime.
I would say I have very little Cal/Carb coming in from runoff, but, it's been a year since I last limed the pond and it's maintained the same Alk level over that period, even after having overflowed multiple times.
The plan is to add another 500# directly to the pond, and 50# of pelletized lime in the inflow ditch from my neighbors property so any future influx of new water coming from there will be picking up some lime on it's way to the pond.
We've had 16" of rain YTD, but the dry season is gonna be here soon.
Thanks for those charts Eric, appreciate that.
About time to do another check of everything, I'll see how it compares.
If pH swings over 24 hours are small then alkalinity is good. It is buffering the swings. Adding lime when needed keeps alkalinity up and it usually lasts (bell curve) about 18 mths depending of course on your dirt.
If pH swings over 24 hours are small then alkalinity is good. It is buffering the swings. Adding lime when needed keeps alkalinity up and it usually lasts (bell curve) about 18 mths depending of course on your dirt.
Thanks for chiming in Eric. I think the level of ALK in the pond has been sufficient to adequately buffer the PH. As I stated earlier, my PH seldom swings much, and if it does it's usually after a hard rain, but never above 8.5 and it comes right back. My main concern is in hardness as I know there's a correlation to overall fish health, and right now, it not even measurable. I may hold off on the 500 additional pounds and just lace the small inflow ditch so any new water is being buffered.
An update here.. Wanted to test pH and alkalinity again and was shocked to see 8.4 pH.
7.5-7.6 is average in this area for the small bodies of water that exist nearby and in a year, I haven't seen above 7.5, until now.
dKH is 9.1= 162.5 ppm.
Will this change with the onset of warmer conditions?
That's what bothers me about adding more aglime. My ph has been sitting pretty in the mid 7s. I'm afraid adding more might raise the ph.
I dont think it should, but in some instances I think it has.
To my understanding there is a max amount that ag lime can raise your Ph. I think it is to 8.2 or 8.5 regardless of how many pounds you put in there. So you can't really over lime a pond. You can waste money putting it in past a certain point.
Hardness is commonly confused
with alkalinity (the total concentration
of base). The confusion relates
to the term used to report
both measures, mg/L CaCO3. If
limestone is responsible for both
hardness and alkalinity, the concentrations
will be similar if not
identical. However, where sodium
bicarbonate (NaHCO3) is responsible
for alkalinity it is possible
to have low hardness and high
alkalinity. Acidic, ground or well
water can have low or high hardness
and has little or no alkalinity.
Agricultural limestone can be used
to increase calcium concentrations
(and carbonate-bicarbonate alkalinity)
in areas with acid waters or
soils. However, at a pH of 8.3 or
greater, agricultural limestone will
not dissolve. Agricultural gypsum
(calcium sulfate) or food grade calcium
chloride could be used to
raise calcium levels in soft, alkaline
waters.
Eric....in your opinion, what is the best source of calcium chloride? (Short of buying a bunch of the real stuff)

