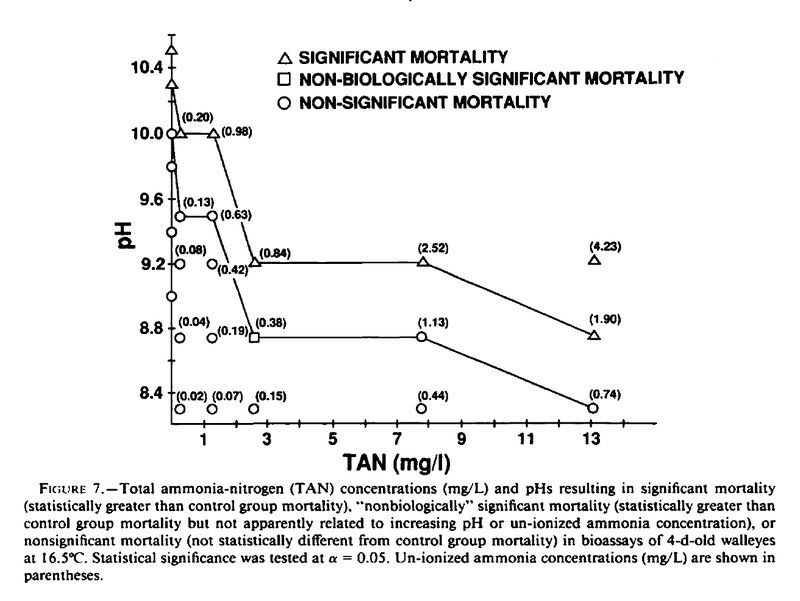
Here is some info wrt your question.
North American Journal of Fisheries Management 12:356-366. 1992
© Copyright by the American Fisheries Society 1992
Lethal Effects of Elevated pH and Ammonia on
Early Life Stages of Walleye
DAVID L. BERGERHOUSE
Cooperative Fisheries Research Laboratory and Department of Zoology
Southern Illinois University. Carbondale. Illinois 62901, USA
Abstract.—Elevated pH may be lethal to larval fish and can affect the toxicity of ammonia. Fry
of walleye Stizostedion vitreum propagated in a controlled hatchery environment are often stocked,
despite little knowledge of their tolerance to high pH or ammonia. A series of 6-h static bioassays
was performed with walleye fry of various ages to determine their tolerance to elevated pH and
the effect of ammonia on pH tolerance. The 6-h mortality threshold pH (the lowest pH at which
significant mortality occurs) was between 10.0 and 10.3 for 3-d-old walleyes, and between 9.8 and
10.0 for both 8- and 12-d-old walleyes, when no measurable ammonia was present. Sublethal
concentrations of un-ionized ammonia increased the toxicity of elevated pH, indicating an interaction
between ammonia and pH. Values of pH that caused statistically significant mortality were
well below values measured in some fish culture ponds and nutrient-rich impoundments. Elevated
pH at the time of stocking walleye fry may result in extensive mortality.
The mortality of 3-d-old walleyes generally increased
as pH increased between values of 10.0
and 10.5. No deaths occurred in bioassays with
no ammonia at pH 9.8 and lower (Figure 1). A
few deaths occurred at pH 10.0, but mortality was
not significantly different from zero. The mortality
at pH 10.3 reached 37% by the end of the 6-h test
and was significantly different from the control
group. Al 3-d-old walleyes died within 120 min
at pH 10.5. A 6-h mortality threshold pH (the
lowest pH at which significant mortality occurs)
for 3-d-old walleyes was therefore estimated to be
between 10.0 and 10.3.
Older walleye fry were slightly less tolerant of
elevated pH than were 3-d-old fry. Mortality at
pH 10.0 increased with age in bioassays containing
3-, 8-, 9-, and 12-d-old fry (Figure 2).