Can we increase hardness WITHOUT increasing pH? - 05/22/19 05:59 AM
I've seen comments about using Please Release Me and other treatments to keep fish healthy. Sea salt is recommended by some folks.
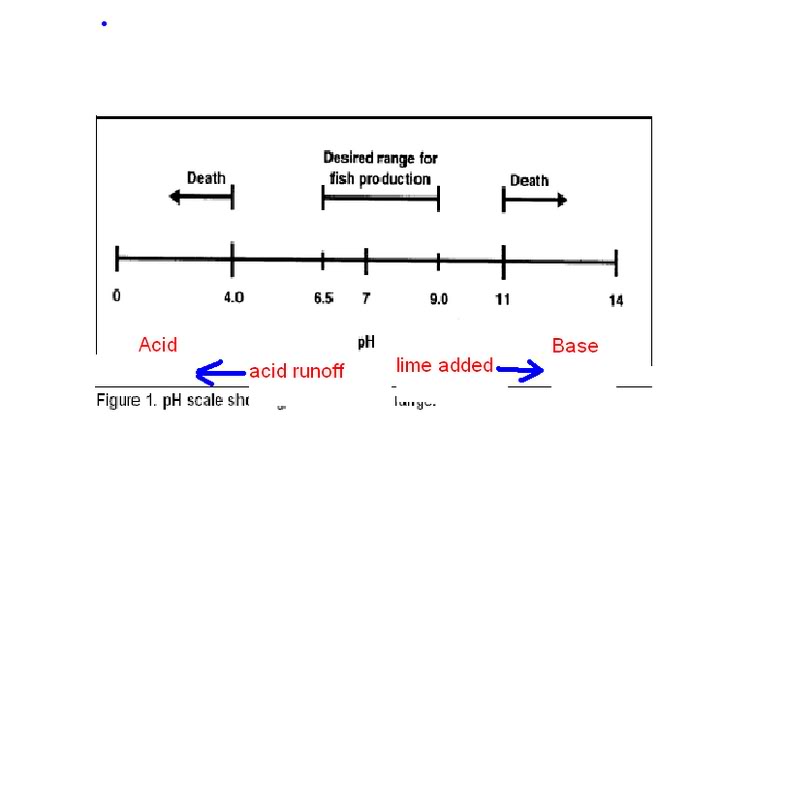
So here's my question: My normally acidic pond now has a pH of 7.8 (I don't know how). However, alkalinity and hardness are down to the 20s, half their usual. My guess is that this is a result of excess rain. I'd like to restore the minerals, but don't know if adding lime would be smart with an already high pH.
Any comments? Would sea salt have any [positive effect? I do have a decent bloom, maybe 30 inch viz, due to a total of 85 lb fertilization, and the fish seem to be doing well.
So here's my question: My normally acidic pond now has a pH of 7.8 (I don't know how). However, alkalinity and hardness are down to the 20s, half their usual. My guess is that this is a result of excess rain. I'd like to restore the minerals, but don't know if adding lime would be smart with an already high pH.
Any comments? Would sea salt have any [positive effect? I do have a decent bloom, maybe 30 inch viz, due to a total of 85 lb fertilization, and the fish seem to be doing well.