Water analysis in...what do you guys recommend?? - 07/25/16 04:03 PM
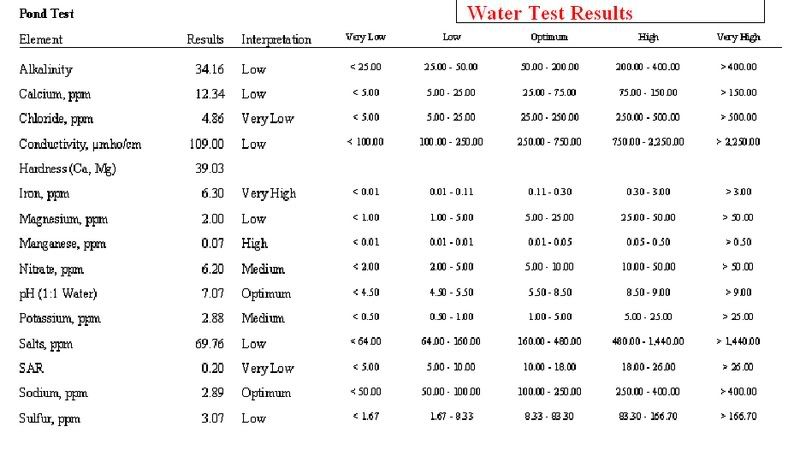
Got my analysis back today. I haven't studied it too much because I'm at work. I think it is as a feared. Very low alkalinity, which has caused pH to drop after each rain. This sample was taken a day or so after a rain. The test strips I was using showed something similar and this analysis verified it.
Looks like I'll be liming in the very near future. Before the rain we had a 2 or 3 weeks of dry weather. At that point my pH was in the low 8's. Since it will go back up...and rain (acidic) makes it go down, will the lime add the alkalinity to buffer and hold my pH at a steadier spot? I'm hoping I'm understanding all my reading so far. Pretty new at this. I still haven't read enough to understand the rest but will be studying more tonight. Even though I'm sure I'll do whatever our experts here tell me.
If anyone else on here has questions to ask the experts feel free to use this thread and this analysis to ask them. Do not feel as if you are hijacking it. You may ask a question that someone else is needing an answer to!
Thanks in advance guys!!
[img:left]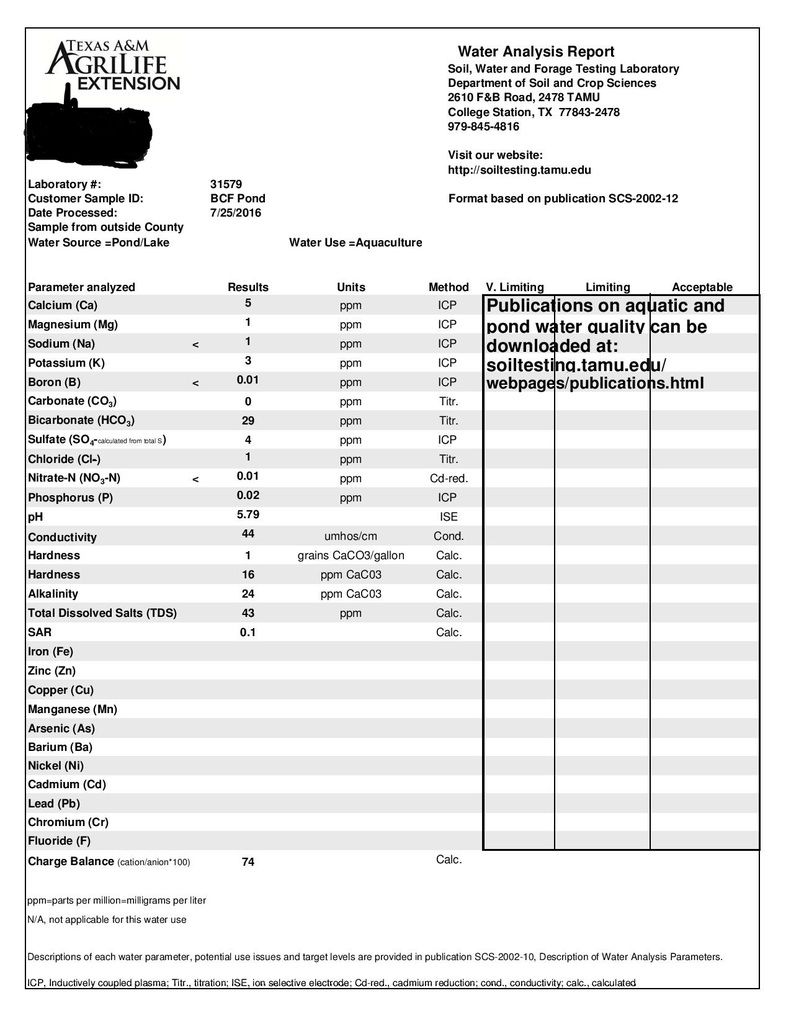 [/img]
[/img]
Looks like I'll be liming in the very near future. Before the rain we had a 2 or 3 weeks of dry weather. At that point my pH was in the low 8's. Since it will go back up...and rain (acidic) makes it go down, will the lime add the alkalinity to buffer and hold my pH at a steadier spot? I'm hoping I'm understanding all my reading so far. Pretty new at this. I still haven't read enough to understand the rest but will be studying more tonight. Even though I'm sure I'll do whatever our experts here tell me.
If anyone else on here has questions to ask the experts feel free to use this thread and this analysis to ask them. Do not feel as if you are hijacking it. You may ask a question that someone else is needing an answer to!
Thanks in advance guys!!
[img:left]
 [/img]
[/img]