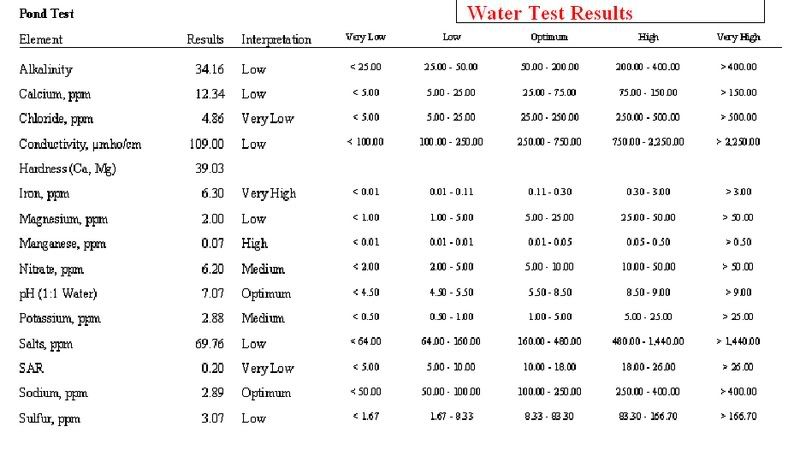
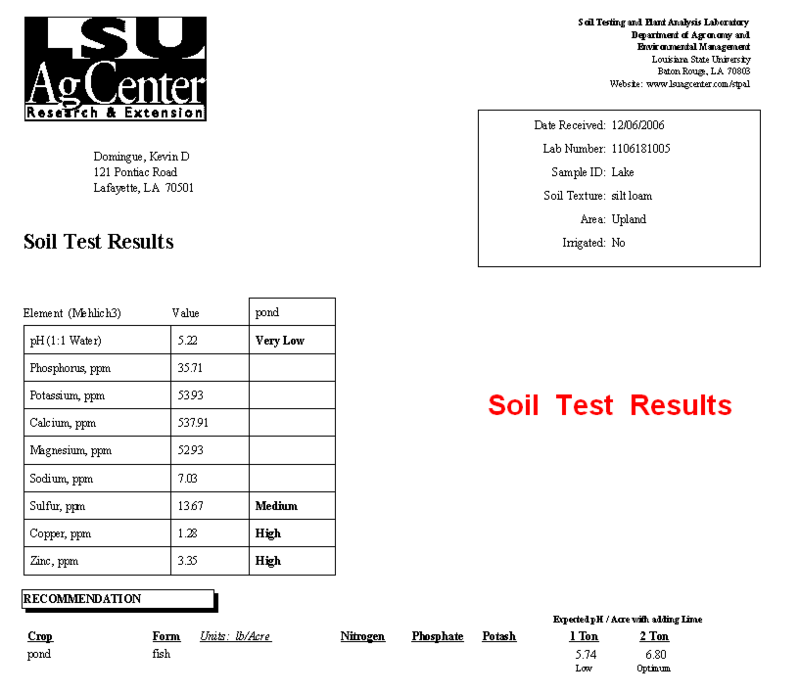
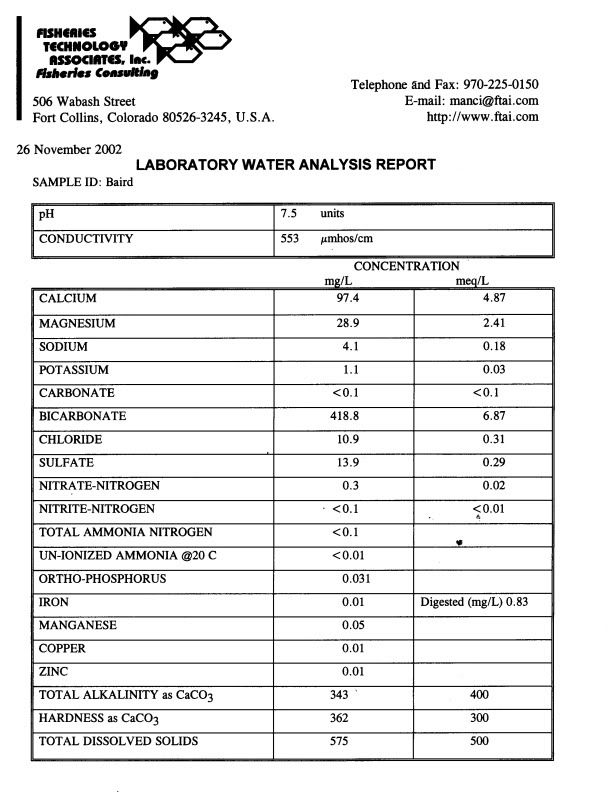
Well water analysis - 05/11/09 04:30 AM
Hello all;
I had a well dug 1.5 years ago before I purchased my land in order to determine whether or not the water table had the necessary GPM to fill and keep my ponds topped off. It turns out we had water - so I purchased the land.
I had enough to pump up to 90 GPM [with a 10 HP pump!] but there existed some risks pumping that high rate from my well over time - so I settled for a smaller pump [5 HP] to handle around 50 GPM.
Bob Lusk pointed out something I never considered in a post today - it's wise to have the chemistry of your well water tested and analyzed prior to commiting to a pond project. Makes sense - I just went with my well driller's assessment that everything in my report "looked fine" [what else would he say?]. Should have brought my test results to the forum before I went and created four ponds, however, I suppose it's still worthwhile to get some feedback from anyone who would be willing to lend their expertise and help me out!
Here are the results:
Nitrate+Nitrate Nitrogren - Not Detected
Flouride - .49 mg/l
ph at 22.0 Celsius - 7.8
Turbidity - 1.0 NTU
Total Dissolved Solids - 483 mg/l
Hardness [CaCO3] - 340 mg/l
Hardness [CaCO3] - 20 grains/gal
Electrical Conductivity - 0.754 mmho/cm
Sulfate [SO4] - 30 mg/l
Total Calcium - 88 mg/l
Total Iron - not detected
Total Magnesium - 0.67 mg/l
Total Sodium - 36 mg/l
Any feedback? I hope these results are within acceptable parameters?
Kinda have a sinking feeling like I'm waiting for a professor to post my grade...I hope I don't have reason to.
Thanks in advance guys...
I had a well dug 1.5 years ago before I purchased my land in order to determine whether or not the water table had the necessary GPM to fill and keep my ponds topped off. It turns out we had water - so I purchased the land.
I had enough to pump up to 90 GPM [with a 10 HP pump!] but there existed some risks pumping that high rate from my well over time - so I settled for a smaller pump [5 HP] to handle around 50 GPM.
Bob Lusk pointed out something I never considered in a post today - it's wise to have the chemistry of your well water tested and analyzed prior to commiting to a pond project. Makes sense - I just went with my well driller's assessment that everything in my report "looked fine" [what else would he say?]. Should have brought my test results to the forum before I went and created four ponds, however, I suppose it's still worthwhile to get some feedback from anyone who would be willing to lend their expertise and help me out!
Here are the results:
Nitrate+Nitrate Nitrogren - Not Detected
Flouride - .49 mg/l
ph at 22.0 Celsius - 7.8
Turbidity - 1.0 NTU
Total Dissolved Solids - 483 mg/l
Hardness [CaCO3] - 340 mg/l
Hardness [CaCO3] - 20 grains/gal
Electrical Conductivity - 0.754 mmho/cm
Sulfate [SO4] - 30 mg/l
Total Calcium - 88 mg/l
Total Iron - not detected
Total Magnesium - 0.67 mg/l
Total Sodium - 36 mg/l
Any feedback? I hope these results are within acceptable parameters?
Kinda have a sinking feeling like I'm waiting for a professor to post my grade...I hope I don't have reason to.
Thanks in advance guys...