|
Forums36
Topics40,944
Posts557,788
Members18,483
| |
Most Online3,612
Jan 10th, 2023
|
|
|
0 members (),
778
guests, and
246
robots. |
|
Key:
Admin,
Global Mod,
Mod
|
|
|
|
Joined: Aug 2014
Posts: 1,381 Likes: 46
|
OP

Joined: Aug 2014
Posts: 1,381 Likes: 46 |
Now can someone decode this for me! haha 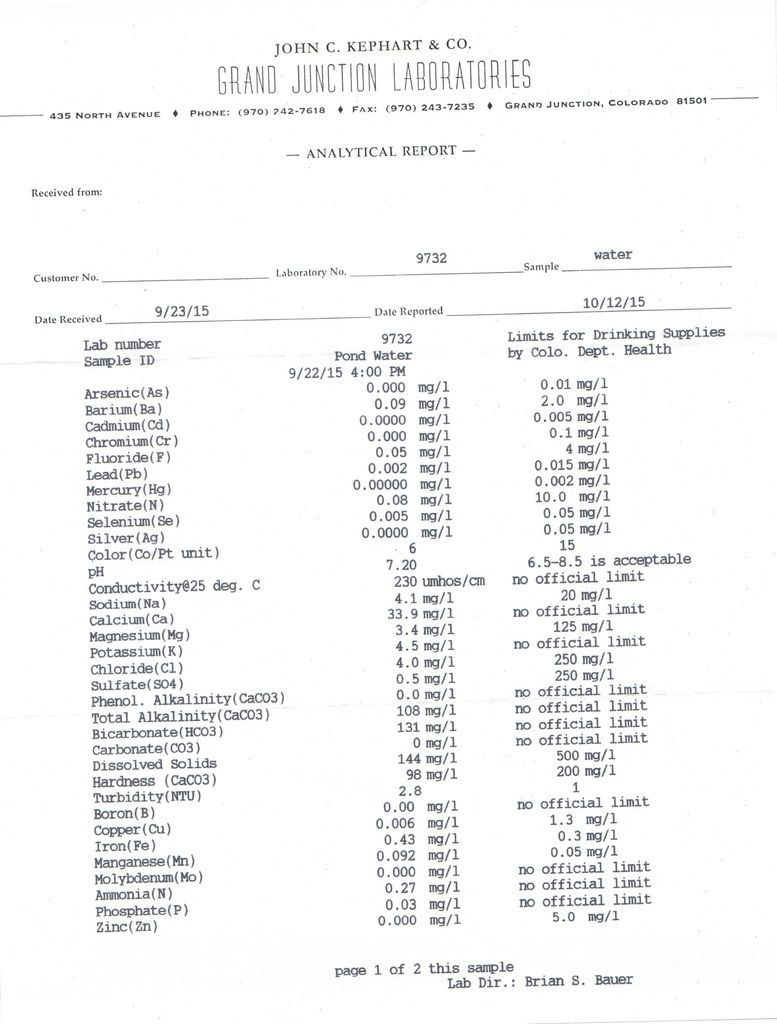
Last edited by wbuffetjr; 10/19/15 02:39 PM.
Keep This Forum Viable, Read Pond Boss Magazine -
America's Journal of Pond Management
|
|
|
|
|
Joined: Apr 2002
Posts: 15,141 Likes: 488
Moderator
Ambassador
Field Correspondent Lunker
|

Moderator
Ambassador
Field Correspondent Lunker
Joined: Apr 2002
Posts: 15,141 Likes: 488 |
Did you have "issues" that prompted the test? Your water chemistry test indicates water is low medium hardness and alkalinity at adequate concentration for producing algae blooms. pH is at the very good 'level'. Dissolved minerals and metals all low and in the normal range. Ammonia of 0.27 is slightly elevated but trout can tolerate 0.2 to 2ppm depending on the pH. Overall very good water quality IMO.
aka Pond Doctor & Dr. Perca Read Pond Boss Magazine -
America's Journal of Pond Management
|
|
|
|
|
Joined: Mar 2005
Posts: 21,490 Likes: 265
Moderator Hall of Fame 2014  Lunker
|

Moderator Hall of Fame 2014  Lunker
Joined: Mar 2005
Posts: 21,490 Likes: 265 |
Many of us can only wish for such good water quality.
|
|
|
|
|
Joined: Aug 2002
Posts: 20,043 Likes: 1
Hall of Fame  Lunker
|

Hall of Fame  Lunker
Joined: Aug 2002
Posts: 20,043 Likes: 1 |
I wonder why the ammonia is 0.27 mg/l?
If pigs could fly bacon would be harder to come by and there would be a lot of damaged trees.
|
|
|
|
|
Joined: Aug 2014
Posts: 1,381 Likes: 46
|
OP

Joined: Aug 2014
Posts: 1,381 Likes: 46 |
Did you have "issues" that prompted the test? No, no issues. I just want to fully understand my pond and its "personality" I guess. Thought this would give me a little more of the total picture. Definitely glad to hear you guys think it is good. From here on, is there anything that I can do to improve it?
Keep This Forum Viable, Read Pond Boss Magazine -
America's Journal of Pond Management
|
|
|
|
|
Joined: Aug 2014
Posts: 1,381 Likes: 46
|
OP

Joined: Aug 2014
Posts: 1,381 Likes: 46 |
I wonder why the ammonia is 0.27 mg/l? What could cause this? If Trout can tolerate .2 to 2ppm, I am definitely all ears!
Keep This Forum Viable, Read Pond Boss Magazine -
America's Journal of Pond Management
|
|
|
|
|
Joined: Aug 2002
Posts: 20,043 Likes: 1
Hall of Fame  Lunker
|

Hall of Fame  Lunker
Joined: Aug 2002
Posts: 20,043 Likes: 1 |
I wonder why the ammonia is 0.27 mg/l? What could cause this? If Trout can tolerate .2 to 2ppm, I am definitely all ears! Good question. In a normal recreational pond you shouldn't have any ammonia readings unless it's extremely fish heavy, plantless and algae free, like a swimming pool. Respectfully i wouldn't want any ammonia reading with trout. Not sure where Bill got those numbers. Trout are extremely sensitive to ammonia. That said, it's the unionized ammonia you need to be concerned with and with your near neutral Ph it's probably quite low. I'm sure that number is Total ammonia NH4 vs. unionized NH3 Could be an error. Doubt your iron reading is correct if you sent it in. Has to be done on site. Got a similar reading to yours from a lab when in reality it was 2.5 mg/l (well water).
Last edited by Cecil Baird1; 10/19/15 09:55 PM.
If pigs could fly bacon would be harder to come by and there would be a lot of damaged trees.
|
|
|
|
|
Joined: Aug 2014
Posts: 1,381 Likes: 46
|
OP

Joined: Aug 2014
Posts: 1,381 Likes: 46 |
I wonder why the ammonia is 0.27 mg/l? What could cause this? If Trout can tolerate .2 to 2ppm, I am definitely all ears! Good question. In a normal recreational pond you shouldn't have any ammonia readings unless it's extremely fish heavy, plantless and algae free, like a swimming pool. Respectfully i wouldn't want any ammonia reading with trout. Not sure where Bill got those numbers. Trout are extremely sensitive to ammonia. That said, it's the unionized ammonia you need to be concerned with and with your near neutral Ph it's probably quite low. I'm sure that number is Total ammonia NH4 vs. unionized NH3 Could be an error. Doubt your iron reading is correct if you sent it in. Has to be done on site. Got a similar reading to yours from a lab when in reality it was 2.5 mg/l (well water). Well that is very interesting interesting because I got a sample tested from the spring that provides water to the cabin as well. We have never drank that water, but we put in a new collection system this year and I wanted to see if it was ok to drink. The Iron came in at 4.49 mg/l and the ammonia at .60 mg/l. This water comes right out of the side of the mountain and once our cistern is full the overflow eventually makes it's way down to our pond.
Last edited by wbuffetjr; 10/20/15 05:40 AM.
Keep This Forum Viable, Read Pond Boss Magazine -
America's Journal of Pond Management
|
|
|
|
|
Joined: Aug 2014
Posts: 1,381 Likes: 46
|
OP

Joined: Aug 2014
Posts: 1,381 Likes: 46 |
Just for comparison, here are the results for the spring. This comes right out of the mountain and eventually makes it to the lake. 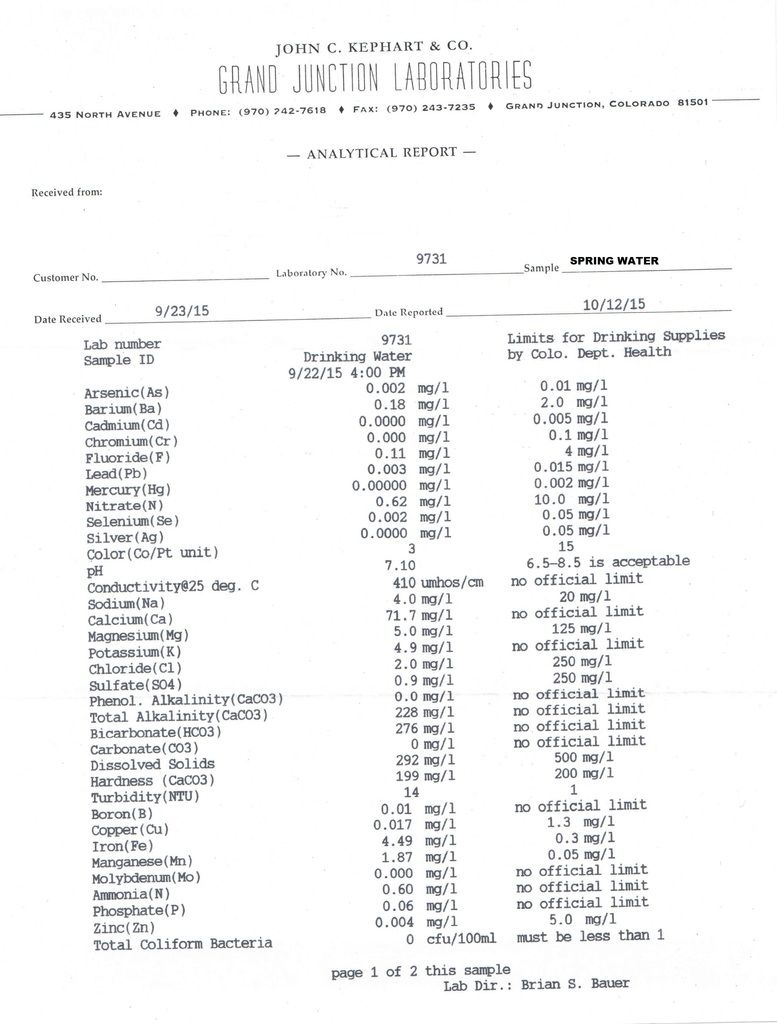 Why would this water have even higher ammonia?
Keep This Forum Viable, Read Pond Boss Magazine -
America's Journal of Pond Management
|
|
|
|
|
Joined: Apr 2002
Posts: 15,141 Likes: 488
Moderator
Ambassador
Field Correspondent Lunker
|

Moderator
Ambassador
Field Correspondent Lunker
Joined: Apr 2002
Posts: 15,141 Likes: 488 |
Note that the main perameters higher for the spring are iron, ammonia, bicoarbonate, alkalinity, hardness, conductivity. CB1 the ammonia range for the trout came from a discussion from an older EPA Quality Criteria for Water. Discussion was probably for total ammonia; uniodized ammonia should always be low as you noted.
Last edited by Bill Cody; 10/20/15 12:51 PM.
aka Pond Doctor & Dr. Perca Read Pond Boss Magazine -
America's Journal of Pond Management
|
|
|
|
|
Joined: Aug 2002
Posts: 20,043 Likes: 1
Hall of Fame  Lunker
|

Hall of Fame  Lunker
Joined: Aug 2002
Posts: 20,043 Likes: 1 |
I wonder why the ammonia is 0.27 mg/l? What could cause this? If Trout can tolerate .2 to 2ppm, I am definitely all ears! Good question. In a normal recreational pond you shouldn't have any ammonia readings unless it's extremely fish heavy, plantless and algae free, like a swimming pool. Respectfully i wouldn't want any ammonia reading with trout. Not sure where Bill got those numbers. Trout are extremely sensitive to ammonia. That said, it's the unionized ammonia you need to be concerned with and with your near neutral Ph it's probably quite low. I'm sure that number is Total ammonia NH4 vs. unionized NH3 Could be an error. Doubt your iron reading is correct if you sent it in. Has to be done on site. Got a similar reading to yours from a lab when in reality it was 2.5 mg/l (well water). Well that is very interesting interesting because I got a sample tested from the spring that provides water to the cabin as well. We have never drank that water, but we put in a new collection system this year and I wanted to see if it was ok to drink. The Iron came in at 4.49 mg/l and the ammonia at .60 mg/l. This water comes right out of the side of the mountain and once our cistern is full the overflow eventually makes it's way down to our pond. If the lower iron level is out of the pond, and not the well, and is correct, that would make sense. That is, the precipitated iron settles out in the pond after oxidation and you get a lower iron reading. Still puzzled by your ammonia levels right out of the ground. If it was me I would purchase an API Aquarium Master water test from Amazon or Ebay (about $20.00) and test on site for ammonia, nitrite, and nitrates, and see if you get the same readings as the lab. Or you could ask your well driller if it's normal to have ammonia readings in your ground water.
Last edited by Cecil Baird1; 10/20/15 01:50 PM.
If pigs could fly bacon would be harder to come by and there would be a lot of damaged trees.
|
|
|
|
|
Joined: Mar 2005
Posts: 21,490 Likes: 265
Moderator Hall of Fame 2014  Lunker
|

Moderator Hall of Fame 2014  Lunker
Joined: Mar 2005
Posts: 21,490 Likes: 265 |
Why are the dissolved solids in the 2nd sample twice that of the first ?
If the water comes out of the mountain anything that can dissolve into water can be in there (minerals from iron to arsenic as well as everything else.
|
|
|
|
|
Joined: Aug 2014
Posts: 1,381 Likes: 46
|
OP

Joined: Aug 2014
Posts: 1,381 Likes: 46 |
Why are the dissolved solids in the 2nd sample twice that of the first ?
after that water fills our cistern the overflow just pours out on the ground and has a few hundred yards to go before it gets to the pond. Just a guess but maybe it is filtered some in between?? I was planning on piping it all the way to the pond, but with the elevated ammonia levels that would not be a good thing (assuming they are accurate)??
Last edited by wbuffetjr; 10/21/15 06:19 AM.
Keep This Forum Viable, Read Pond Boss Magazine -
America's Journal of Pond Management
|
|
|
|
|
Joined: Mar 2005
Posts: 21,490 Likes: 265
Moderator Hall of Fame 2014  Lunker
|

Moderator Hall of Fame 2014  Lunker
Joined: Mar 2005
Posts: 21,490 Likes: 265 |
I thought the 1st test was from the pond and the second test from the spring ???
|
|
|
|
|
Joined: Aug 2014
Posts: 1,381 Likes: 46
|
OP

Joined: Aug 2014
Posts: 1,381 Likes: 46 |
yes that is correct. The numbers are higher in the sample coming out of the spring.
EDIT: To clarify, I thought dissolved solids were minerals and such that came from the rocks where the water originated. I assumed some of that could be "filtered out" when that water comes to the surface and flows through soil, pine needles, etc before it gets to the pond. I figred maybe that was happening with the Iron, Ammonia and other items that showed up in lower amounts in the pond water.
Last edited by wbuffetjr; 10/21/15 02:54 PM.
Keep This Forum Viable, Read Pond Boss Magazine -
America's Journal of Pond Management
|
|
|
|
|
Joined: Mar 2005
Posts: 21,490 Likes: 265
Moderator Hall of Fame 2014  Lunker
|

Moderator Hall of Fame 2014  Lunker
Joined: Mar 2005
Posts: 21,490 Likes: 265 |
Was sample 2 directly from the spring or from the ground after it ran over the dirt/land ?
Total dissolved solids (TDS) is a measure of the combined content of all inorganic and organic substances contained in a liquid in molecular, ionized or micro-granular (colloidal sol) suspended form. Generally the operational definition is that the solids must be small enough to survive filtration through a filter with two-micrometer (nominal size, or smaller) pores. Primary sources for TDS in receiving waters are agricultural and residential runoff, leaching of soil contamination and point source water pollution discharge from industrial or sewage treatment plants. The most common chemical constituents are calcium, phosphates, nitrates, sodium, potassium and chloride, which are found in nutrient runoff, general stormwater runoff and runoff from snowy climates where road de-icing salts are applied. The chemicals may be cations, anions, molecules or agglomerations on the order of one thousand or fewer molecules, so long as a soluble micro-granule is formed. More exotic and harmful elements of TDS are pesticides arising from surface runoff. Certain naturally occurring total dissolved solids arise from the weathering and dissolution of rocks and soils. The United States has established a secondary water quality standard of 500 mg/l to provide for palatability of drinking water.
Last edited by ewest; 10/21/15 04:51 PM.
.gif) 
|
|
|
|
|
Joined: Aug 2014
Posts: 1,381 Likes: 46
|
OP

Joined: Aug 2014
Posts: 1,381 Likes: 46 |
If it was me I would purchase an API Aquarium Master water test from Amazon or Ebay (about $20.00) and test on site for ammonia, nitrite, and nitrates, and see if you get the same readings as the lab.
Or you could ask your well driller if it's normal to have ammonia readings in your ground water.
Cecil I will definitely be doing my own Ammonia test to confirm. Unfortunately I won't be back out there till next summer so that sucks. I don't have a well driller, but I will definitely start asking around! Thanks!
Keep This Forum Viable, Read Pond Boss Magazine -
America's Journal of Pond Management
|
|
|
|
|
Joined: Aug 2014
Posts: 1,381 Likes: 46
|
OP

Joined: Aug 2014
Posts: 1,381 Likes: 46 |
Was sample 2 directly from the spring or from the ground after it ran over the dirt/land ? I took the water right out of the end of the overflow pipe so as direct from the spring as I can get it.
Keep This Forum Viable, Read Pond Boss Magazine -
America's Journal of Pond Management
|
|
|
|
|
Joined: Aug 2002
Posts: 20,043 Likes: 1
Hall of Fame  Lunker
|

Hall of Fame  Lunker
Joined: Aug 2002
Posts: 20,043 Likes: 1 |
http://www.saskh20.ca/DWBinder/epb431.pdfhttp://scholarworks.wmich.edu/cgi/viewcontent.cgi?article=1452&context=masters_theses&sei-redir=1&referer=http%3A%2F%2Fwww.google.com%2Furl%3Fsa%3Dt%26rct%3Dj%26q%3Dnaturally%2Boccurring%2Bammonia%2Bin%2Bgroundwater%26source%3Dweb%26cd%3D3%26ved%3D0CCIQFjACahUKEwjbjufe49XIAhWM4SYKHWWZDig%26url%3Dhttp%253A%252F%252Fscholarworks.wmich.edu%252Fcgi%252Fviewcontent.cgi%253Farticle%253D1452%2526context%253Dmasters_theses%26usg%3DAFQjCNEOWzne9GqWTr8VRroLjeGK6hFCjw#search=%22naturally%20occurring%20ammonia%20groundwater%22
Last edited by Cecil Baird1; 10/22/15 04:41 AM.
If pigs could fly bacon would be harder to come by and there would be a lot of damaged trees.
|
|
|
|
|
Joined: Aug 2014
Posts: 1,381 Likes: 46
|
OP

Joined: Aug 2014
Posts: 1,381 Likes: 46 |
That is pretty interesting
Keep This Forum Viable, Read Pond Boss Magazine -
America's Journal of Pond Management
|
|
|
Moderated by Bill Cody, Bruce Condello, catmandoo, Chris Steelman, Dave Davidson1, esshup, ewest, FireIsHot, Omaha, Sunil, teehjaeh57 |
|