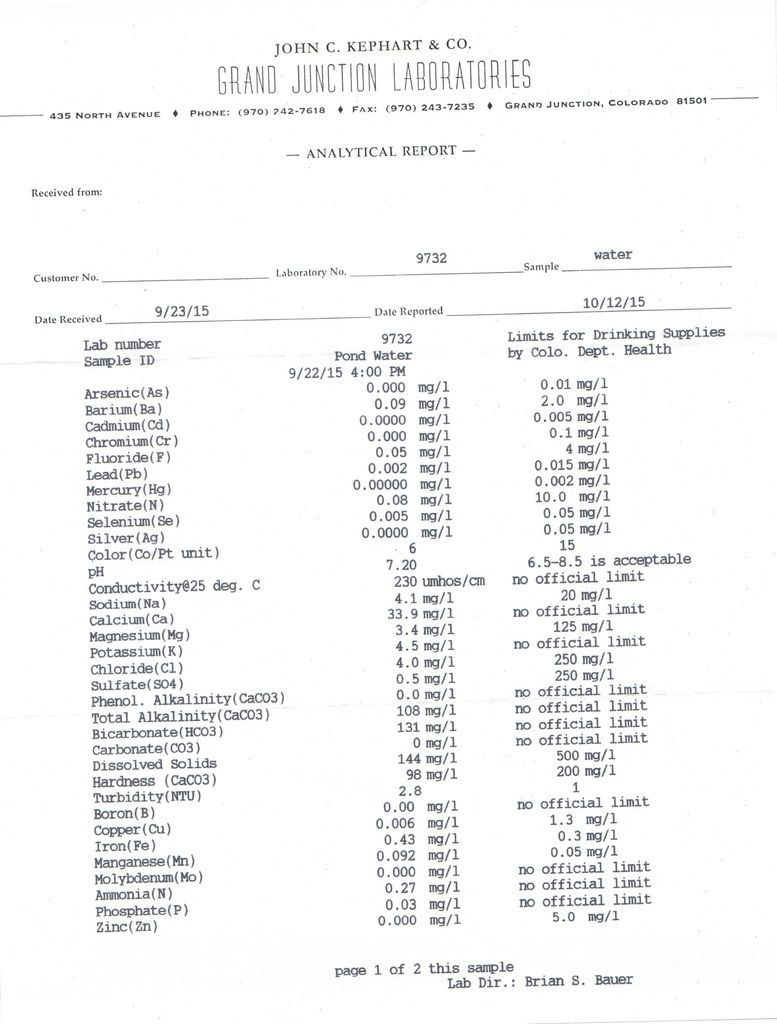
I wonder why the ammonia is 0.27 mg/l?
What could cause this? If Trout can tolerate .2 to 2ppm, I am definitely all ears!
Good question. In a normal recreational pond you shouldn't have any ammonia readings unless it's extremely fish heavy, plantless and algae free, like a swimming pool.
Respectfully i wouldn't want any ammonia reading with trout. Not sure where Bill got those numbers. Trout are extremely sensitive to ammonia. That said, it's the unionized ammonia you need to be concerned with and with your near neutral Ph it's probably quite low. I'm sure that number is Total ammonia NH4 vs. unionized NH3
Could be an error. Doubt your iron reading is correct if you sent it in. Has to be done on site. Got a similar reading to yours from a lab when in reality it was 2.5 mg/l (well water).
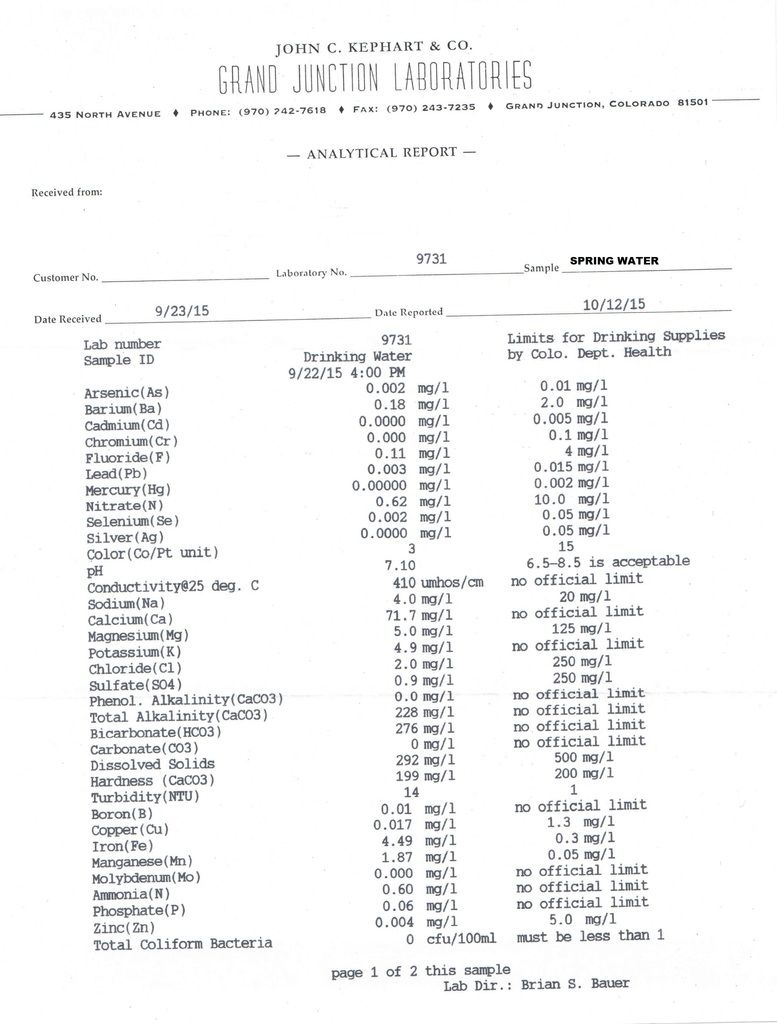
Well that is very interesting interesting because I got a sample tested from the spring that provides water to the cabin as well. We have never drank that water, but we put in a new collection system this year and I wanted to see if it was ok to drink.
The Iron came in at 4.49 mg/l and the ammonia at .60 mg/l. This water comes right out of the side of the mountain and once our cistern is full the overflow eventually makes it's way down to our pond.
If the lower iron level is out of the pond, and not the well, and is correct, that would make sense. That is, the precipitated iron settles out in the pond after oxidation and you get a lower iron reading.
Still puzzled by your ammonia levels right out of the ground.
If it was me I would purchase an API Aquarium Master water test from Amazon or Ebay (about $20.00) and test on site for ammonia, nitrite, and nitrates, and see if you get the same readings as the lab.
Or you could ask your well driller if it's normal to have ammonia readings in your ground water.